John Tolley, June 1, 2019
What if the fuel of the future didn?t just run clean, but actively took pollutants right out of our environment? This scenario is looking a lot more likely thanks to a recent discovery by a team of Northwestern University researchers.
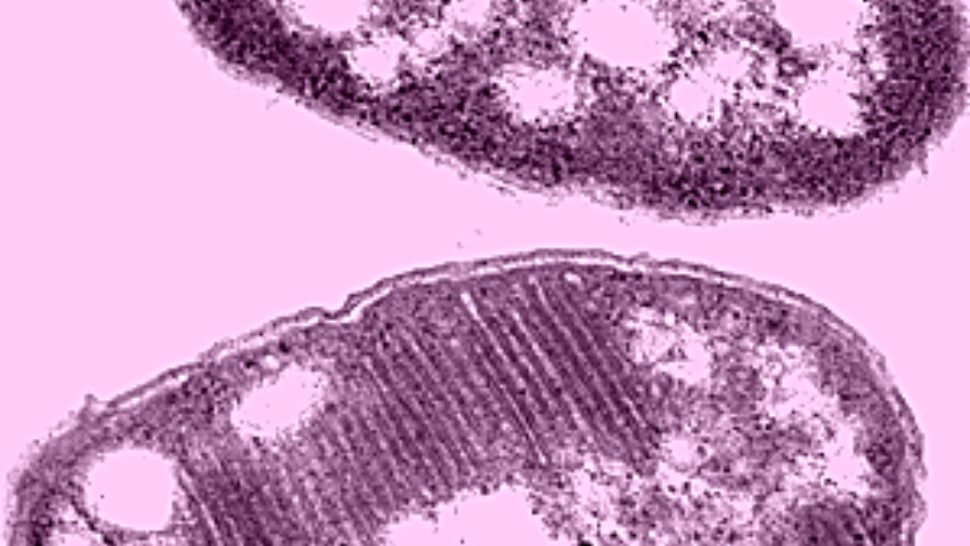
It all hinges on ravenous methanotrophic bacteria, which absorb environmental methane, a top greenhouse gas, and converts it to methanol, a usable fuel source. But the conversion process has long flummoxed scientists. Recently though, a study, published in the journal Science, revealed that a solitary copper ion located in the catalysis reaction sight is the key to the process.
Speaking with Northwestern University News, study co-senior author Brian M. Hoffman, the Charles E. and Emma H. Morrison Professor of Chemistry at Northwestern?s Weinberg College of Arts and Sciences, illuminated the gravity of the discovery.
?By identifying the type of copper center involved, we have laid the foundation for determining how nature carries out one of its most challenging reactions.?
Methanotrophic bacteria hold distinct advantages over the current methanol conversion processes. At the industrial scale, these operations require extremes of both pressure and heat, and therefore no small amount of energy. The bacteria, on the other hand, need neither, converting methane naturally.
The study?s authors, which include Amy C. Rosenzweig, the Weinberg Family Distinguished Professor of Life Sciences, are hopeful that their discovery will lead to optimized catalyzation processes that can feasibly provide fuel while scrubbing the environment.
To learn more about Northwestern?s commitment to sustainability and environmental action, check out the SustainNU website.