Eli Kaberon, December 8, 2017
Drug manufacturers have long used animals as test subjects in a ?lesser of two evils? approach. According to the Federal Drug Administration, animal testing is only done when there is concern for the drug?s ability to function with living tissue without harming the tissue itself. The testing is done generally on fish, mice and pigs in a controlled setting, allowing medical professionals to closely document results without harming the animals themselves.
But what if there was safer way to test for impact, interactions and side effects?
Purdue University professor of mechanical and biomedical engineering Bumsoo Han is attempting to develop an alternative to animal testing, while still providing drug companies the ability to study their products and earn government compliance before they reach the public marketplace.
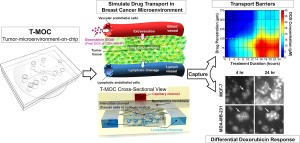
Working specifically to improve the development of cancer treatments, Han created a technology that simulates tumors, giving drug manufacturers a way to review their products without needing animals for testing. The device?s purpose is to incorporate biopsied cancer cells from patients in order to evaluate the effectiveness of different drugs.
?As of now most of the response has come from the drug discovery,? Han said, noting that manufacturers ask to use the technology to determine the proper drug combination prior to going to a clinical trial. ?(Manufacturers) actually see the benefit of this model. Several researchers have actually approached us to see if they can test their drug compounds on our device before they actually try it on the animals, so that they can reduce the number of experiments on animals.?
Han?s device is called T-MOC, which stands for tumor-microenvironment-on-chip. T-MOC doesn?t take up much space - it is just 4.5 centimeters square - but it can have powerful results. With so many different types of chemotherapy drugs on the market, finding the right one to combat specific types of cancers can be difficult. What Han has created has the ability to assist clinicians, helping them narrow down the drugs that will be more effective than their counterparts. And it is all done without needing to research or test on animals.
In November, Han and Purdue postdoctoral research associate Altug Ozcelikkale released some of their findings in the Journal of Controlled Release. The pair detailed how T-MOC could possibly be used for precision medicine, giving individual patients access to specifically tailored treatments. The device also is able to replicate what?s called ?plasma clearance,? which is when the body starts to recognize specific drugs, filtering only some of the medicine towards the tumor. These obstacles are complicated to duplicate in animal testing, but are important for drug manufacturers.
That differentiator is a major aspect of the next steps Han hopes to take with T-MOC moving forward. He and his team are focusing on a specific type of cancer, hoping that their breakthrough can start to save lives.
The Institute of Health, one of the foundations that helped fund Han?s research, has already expressed an interest in the device moving forward.
?So as of now, we are going in two different directions,? Han said. ?One is that the we are trying to separate ourselves from the human patient, so that we can mimic the human tumor better. And second, we are starting to focus on the pancreatic cancer, because it is one of the most severe. The survival rate is only about 7 to 8 percent, and it hasn't been improved for 30 to 40 years. So we've been trying to mimic the pancreatic cancer so that the primary researcher can mimic it to develop those types of drugs.?